If you have been following our WeInfuse IV Insights Blog for a while, then you are already familiar with our previous articles about billing for infusion and injectable products in the physician’s in-office infusion suite and in stand-alone Infusion Centers. If you have not already, be sure to review our previous blog entries before moving on. Start with Billing for Infusion Time – “The Confusion of Infusion Billing” then HCPCS Billing for Medications – “Billing Infusion Medications” as pre-requisites to this guide.
For this article, I will use the term “infusion billing” to generically describe billing for any injectable product – infusion (IV), intramuscular (IM), subcutaneous (SQ), etc. In this writing, I will cover the process and my best understanding of the NDC Billing Format.
Background – NDC Numbers
NDC stands for “National Drug Code” and is currently an 11 digit segmented numeric value assigned to all drugs that are “manufactured, prepared, propagated, compounded, or processed…for commercial distribution.” – from FDA’s NDC Webpage NDCs are typically segmented and separated by hyphens to help with their identification.
50242-0137-01 | <-- 11-digit NDC number for Genentech’s Actemra 400mg vial |
|---|
50242 | – | 0137 | – | 01 |
|---|---|---|---|---|
Labeler | – | Product | – | Package Code |
Genentech | – | Actemra | – | 400mg (20ml) Vial |
The first five digits identify the manufacturer (labeler) of the drug and are assigned by the FDA. The remaining digits are assigned by the manufacturer and identify the specific product and package size. The 11 digit NDC format is the newest format which means there are still many 10 digit NDC numbers floating around in the market. As we will cover in more detail, it is important to convert any 9 or 10 digit NDC number to an 11 digit NDC for proper NDC billing.
XXXX-XXXX-XX = 0XXXX-XXXX-XX
XXXXX-XXX-XX = XXXXX-0XXX-XX
XXXXX-XXXX-X = XXXXX-XXXX-0X
To convert a 10 digit NDC number into the correct 11 digit format, you simply add a zero in the correct location as shown in the above guide. This is easy if you have the hyphenated NDC number as shown. If not, you will have to do some “googling” or reach out to the manufacturer of the product for help. (Or, you can use WeInfuse and we do this conversion for you)
What is “NDC Billing”?
Now that you have a good primer on NDCs, we can move onto NDC billing. When I refer to NDC billing, I am speaking specifically about the NDC billing requirement for physician’s offices and stand-alone Infusion Centers who bill for medications through a patient’s medical benefit.
There is an important difference between simply putting the NDC number of a medication on a claim form vs. the actual NDC billing process. Many offices I work with have started adding the medication NDC number on their claims without any additional qualifier or NDC units. While this is helpful and possibly required by some payers, this is not the NDC billing we are discussing here.
NDC billing follows a specific process that involves including the “N4” qualifier, the 11-digit medication NDC number, and the unit/basis value in a specific format in specific places on your claims.
Why is NDC Billing Important?
The NDC billing process is now required by many large commercial payers and state Medicaid plans. More commercial payers will be mandating NDC billing moving forward in order to more accurately identify and reimburse providers for the exact product used for a patient’s treatment. Having the specific NDC of the medication allows payers to avoid some of the guesswork involved for reimbursing using the HCPCS and billing Unit process that we all know and love. In addition, having the specific NDC on the claim helps payers navigate the issues associated with the newly approved and “not otherwise classified” medications often billed with J3490 and J3590 HCPCS codes.
In January 2017, United Healthcare released a memo outlining their mandate requiring the NDC billing format on all physician claims. Many other payers such as Blue Cross Blue Shield of Texas allow, but do not currently mandate, the NDC billing format on claims. For these payers, NDC billing allows the provider to use the NDC Fee Schedule that is updated monthly. Unlike the traditional HCPCS fee schedule which is updated quarterly. Typically, the NDC fee schedule will result in increased reimbursements as price increases for different products are updated more timely. In short, if you are not having to process claims using the NDC billing format today, you will soon.
At this point, if you have decided that you just want a software system that will take care of most this complexity for you and your office team, skip down to the very end and we will tell you about one, in particular, we are very proud of.
NDC Billing Process
A claim with correctly formatted NDC billing values will have the correct NDC billing format (described below) placed into the line 24 section for the CMS-1500 and a combination of positions and values in the 837. Most revenue cycle/billing software will have provisions to make sure this information is encoded correctly. In addition to the NDC billing format, you also need to include the correct HCPCS code and HCPCS billing units.
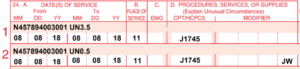
Above: Example CMS-1500 form. The NDC billing information is shown formatted for Remicade 100mg vial in the red shaded area on each claim line. Notice how the line is split into 2 lines to indicate wastage for this treatment.
NDC billing is a format of applying the right values in the right claim fields. This formatting requires the following information on a claim:
- N4 qualifier
- 11-digit NDC number
- NDC Unit of Measure
- NDC Units administered/used
I will break these down in detail, but here is the final format below as a guide. We will use Genentech’s Actemra 400mg vial as an example.
N450242013701 ML20 | <-- NDC Formatted Value |
|---|
N4 | 50242013701 | ML | 20 |
|---|---|---|---|
Qualifier | 11-digit NDC | NDC Unit of Measure | NDC Billing Unit |
N4 Qualifier
The CMS-1500 form and the HIPAA 837 electronic claim form were not originally designed to handle billing of medications effectively. In the case of NDC billing, the industry has designated the “N4” value as the qualifier to tell these systems that the next values that follow are NDC values. All NDC billing I have seen to date requires the N4 qualifier to precede the 11-digit NDC number in order to process the claim correctly.
11-digit NDC Number
As covered in detail earlier, an 11-digit NDC number without any spaces or hyphens is required to immediately follow the N4 qualifier.
NDC Unit of Measure
The NDC Unit of Measure (and the NDC Unit covered next) has nothing to do with our current understanding of HCPCS billing units. There are a total of 5 recognized NDC Units of Measure for NDC billing, but only 2 of them apply here so we won’t discuss the other 3.
NDC Billing Units
This is the hard part for those billers new to NDC billing. I cannot stress enough to forget what you know about HCPCS billing units when calculating NDC billing units. Yes, sometime coincidentally these values may be the same, but that assumption will have you correcting many claims. It is best to think of the HCPCS billing units and the NDC billing units as completely different concepts.
NDC Billing Units are based on which NDC Unit of Measure is used for the medication on the claim.
ML
For medications supplied in a vial or in liquid form. For “ML” you need to know the total volume of medication in the container (vial, bag, syringe, ampul, etc). It can be confusing since many medication vials are labeled in MG or G so you need to look closely for the ML volume. In our Actemra 400mg vial example above, the vial is in liquid form and contains 20mls. For every 400mg vial of Actemra used, we will have an ML20 value.
UN
For medications supplied in a vial/container in powder form which must be reconstituted before administration. For “UN” the units are much simpler as it is just 1 unit of UN per vial or container. Remicade (infliximab) comes packaged in a 100mg lyophilized (powder) vial and uses UN as the NDC unit of measure. For each individual vial of Remicade used on a claim, we will have a UN1 value. If 2 vials were used 2 = UN2, 3 = UN3 and so on.
If you are looking at the medication vial or bag and you see liquid, then use “ML.” If you see powder, then use “UN.”
NDC Billing for Wastage
For infusion billing in the physician infusion suite, accounting for wastage on claims is common. NDC billing requirements complicate billing for wastage as you must account for the wastage both in the HCPCS and in the NDC billing units.
The wastage will have its own claim line, just like in standard HCPCS billing, but the wastage units will be listed for both the HCPCS billing unit wastage and NDC billing unit wastage. This claim line will also have the standard JW modifier. For NDC billing units, you are allowed to use decimals, which is different from HCPCS billing units where only whole numbers are allowed.
Here is an example using 4 Remicade 100mg vials where the amount administered is 350mg with 50mg of wastage documented on the nurse’s treatment note
Code | Unit | Modifier | Line Note |
|---|---|---|---|
J1745 | 35 | N457894003001 UN3.5 |
|
J1745 | 5 | JW | N457894003001 UN0.5 |
Putting it all Together
So now that you have all the pieces of the NDC billing puzzle, let’s move on to apply all these concepts. I am assuming at this point that you have a solid revenue cycle software and that you have done the required research to know where to put all the values into the system to get the NDC formatted and located correctly on your electronic claims. You will likely need to speak directly with your billing software provider and/or claims clearinghouse to confirm this information is showing up correctly on your 837.
Most billing software will have a similar layout for line item entry on a claim that will look roughly like the claim line item example below. In addition, they will likely have fields for diagnosis (ICD-10), charge, expected, and a few other columns of information. For our example, we will look at the values for Code (HCPCS), Unit, Modifier, and Line Note
Code | Unit | Modifier | Line Note |
|---|
Let’s look at a treatment note for a patient who received 528mg of Actemra IV – Buy&Bill, administered over 1 hour. According to the infusion nurse’s treatment note, the inventory used was 2 vials of Actemra 80mg and 1 vial of Actemra 400mg for a total of 560mg’s selected from inventory. Based on both the administered amount of 528mg, we can determine that 32mg of Actemra was wasted.
Based on the treatment documentation, the NDC billing format for this date of service would result in the following line items and values on this claim:
Code | Description | Unit | Modifier | Line Note |
|---|---|---|---|---|
96413 | High Level Infusion 1st Hour | 1 | ||
J3262 | Actemra 80mg/4ml vial | 128 | N450242013501 ML6.4 |
|
J3262 | Actemra 80mg/4ml vial | 32 | JW | N450242013501 ML1.6 |
J3262 | Actemra 400mg/20ml vial | 400 | N450242013701 ML20 |
Practice Implications of NDC Billing
As you can hopefully see now after reading the information contained in this article, NDC Billing is going to complicate your current infusion billing workflow. A successful NDC billing process will require that your infusion clinical documentation be sufficient enough to capture the individual medication vials and the specific NDCs used on each individual patient treatment. The updated treatment documentation should clearly indicate the exact amount of medication administered and wasted for the specific date of service being billed. In addition to the need to improve clinical documentation, the sheer amount of additional information needed to enter into each claim line is going to tax billing resources. Infusion billing teams will need to have solid resources for determining the exact NDCs (Individual Vial and Package NDCs) of your medications, and also have resources for determining NDC Units of Measure and NDC Billing Units for each medication.
NDC Unit Conversion Factors
Unfortunately, I have not found a reliable, updated, and free resource for determining both the NDC Units of Measure and NDC Billing Units for individual medication containers. The vast variety and count of new and existing NDCs for specialty medications and pre-medications such as diphenhydramine, methylprednisolone, etc. make it very difficult to keep updated lists for every possible NDC you might see in your practice’s infusion suite. There are various conversion tables published online by state Medicaids and other commercial payers monthly, but I consistently find these tables to contain errors with regards to the proper NDC Billing Units of Measure and NDC Billing Units.
These tables contain a Conversion Factor, which when multiplied by the HCPCS Billing Units for the medication, will give you the NDC Billing Units for that particular NDC. These tables typically include the NDC Billing Unit of Measure for “UN” or “ML” for each specific NDC as well. If you can’t find the conversion factors table for the payer you are billing, you can determine this yourself by looking up the specific medication NDC to determine if it is a liquid (ML) or powder (UN) in its original package form, then divide the total volume of MLs by the HCPCS Billing Unit for that particular medication to get the conversion factor for that particular NDC.
Actemra (Liquid ML) Example: The Actemra 400mg we used earlier is a 20ml liquid vial so we will use “ML” as our NDC Unit of Measure. If we divide the liquid volume of the vial 20ml by the HCPCS Billing Unit for Actemra which is “1” then we get a conversion factor of “20” (20/1 = 1)
Remicade (Powder UN) Example: A Remicade 100mg vial is a lyophilized powder that must be reconstituted before administration, so we use “UN” as our NDC Unit of Measure. For UN vials, we always use “1” divided by the HCPCS Billing Unit for the medication. For Remicade 100mg, the conversion factor is “0.1” (1/10 = 0.1)
Here are a few NDC Unit Conversion Factors for some common biologics:
Medication | NDC | HCPCS Code | HCPCS Billing UOM/Unit | NDC Unit of Measure | NDC Billing Units Per Vial | NDC Conversion Factor |
|---|---|---|---|---|---|---|
Orencia 250mg/15ml vial | 00003218710 | J0129 | 10mg | UN | 1 | 0.04 |
Tysabri 300mg/15ml vial | 64406000801 | J2323 | 1mg | ML | 15 | 0.05 |
Ocrevus 300mg/10ml vial | 50242015001 | J2350 | 1mg | ML | 10 | 0.0333 |
Simponi ARIA 50mg/4ml vial | 57894035001 | J1602 | 1mg | ML | 4 | 1 |
Conclusion
I hope this latest blog entry has given you and your billing teams a better understanding of the requirements for proper formatting for NDC Billing on your claims. I cannot stress enough that understanding and mastering the NDC billing format is going to be critical for the financial success of your in-office infusion suite or stand-alone Infusion Center. Every commercial payer survey I have seen in the past year has listed “NDC Billing” and “Capturing Specific NDC information on claims” as one of the top strategies they are considering for improving data capture and medication utilization controls in the coming years. I am not convinced that your EMR/EHR or RCM system will be updating their interfaces soon to help your billing teams with this task. Unless your office is already using an oncology-based software, most EHR vendors are not equipped to understand the complexities of billing medications on medical benefit claims. Even if they did, since most EHRs do not include inventory management modules and therefore the software won’t have the information needed to make these complicated calculations.
The good news is that WeInfuse DOES NDC BILLING! WeInfuse does have the required Treatment Documentation and Inventory Management modules to properly determine the exact NDC, NDC Unit of Measure, and NDC Billing Units that you need to properly format and bill your infusion claims. Proper NDC Billing values are automatically determined by WeInfuse the moment the infusion nurse completes the treatment documentation. Stop asking your infusion nurses to complete additional paper superbills for each patient treatment. Let your infusion nurses focus on patient care and your infusion biller to focus on insurance billing.
WeInfuse determines exactly what can be billed directly from the nurse’s treatment documentation. Reduce your audit risk, increase your billing accuracy, and reduce the paperwork on your entire infusion team by implementing WeInfuse in your in-office infusion suite or stand-alone Infusion Center.
If you would like to learn more about how our WeInfuse infusion workflow software can help “Take the Confusion out of Infusion” for your in-office infusion suite or stand-alone Infusion Center, click here to request a WeInfuse Demo Let’s start a conversation about how WeInfuse can help you and your team Infuse Better.
Disclaimer: This article is meant as an instructional guide and is in no way intended to be formal billing advice. WeInfuse always recommends consulting your CPT and HCPCS manuals and/or other applicable reference materials from your payers directly for specific billing requirements.