There’s much at stake if you are directly or indirectly responsible for moving medications from the manufacturer to a patient. Bryan Johnson goes in depth about the DSCSA and leveraging barcodes that are a part of everyday operations in the healthcare supply chain.
For most of us who have embraced the self-checkout option at our local grocery store, barcodes are something we understand at a basic level. I don’t believe the average person moving a product across the laser is aware of the technology powering this process, but we all perceive that some kind of magic is happening to help the checkout machine know the difference between a bag of spicy nacho cheese Doritos and a gallon of milk.
If you or your team are involved in one of the many healthcare supply chain markets and you are directly or indirectly responsible for moving medications from the manufacturer to a patient, then there is much more at stake in your world than your groceries.
Before we begin demystifying barcodes for medications, we need to understand why these barcodes appear on our medications in the first place. For the purpose of this article, we are talking about the 2D GS1 Datamatrix barcodes that appear on medications as a result of the Drug Supply Chain Security Act.
What is the DSCSA?
The Drug Supply Chain Security Act (DCSCA) is part of the Drug Quality and Security Act (DQSA), enacted by Congress on November 27, 2013. Title II of the DQSA, the Drug Supply Chain Security Act (DSCSA), outlines steps to achieve interoperable, electronic tracing of products at the package level to identify and trace certain prescription drugs as they are distributed in the United States. If you have heard of the phrase “track and trace” before, this is where that reference comes from.
“The DSCSA was enacted in 2013 to further secure our nation’s drug supply. It creates a tighter, closed prescription drug distribution system to prevent harmful drugs from entering the supply chain, harmful drugs if they do enter the supply chain, and enable rapid response when such drugs are found.”
In summary, the DQSA was an act of Congress that created the DSCSA and many other new regulations. The act laid out a multi-year implementation plan with various milestones along its timeline. We are now coming up on one of the last deadlines of DSCSA, which is full system interoperability for unit-level tracing by November 2023. You can read all about it on the FDA’s webpage and we will link to additional resources as we go.
 Why Do We Need the DSCSA?
According to the FDA, the DSCSA was created to…
Why Do We Need the DSCSA?
According to the FDA, the DSCSA was created to…
Improve detection and removal of potentially dangerous drugs from the drug supply chain to protect U.S. consumers.
Establish national licensure standards for wholesale distributors and third-party logistics providers, and require these entities to report licensure and other information to the FDA annually.
In short, the act should help everyone in the supply chain from the manufacturer to the individual patient. Most of this is done by more securely controlling the distribution (licensure) and monitoring (track and trace) of the medication supply chain.
In addition, the DSCSA has provisions and requirements that should also allow you to improve patient safety and help with inventory management.
Why Should You Care About the DSCSA?
If you are reading this article as a manufacturer, wholesaler, repackager, or dispenser, then according to the FDA, you are required to comply with the provisions of the act including the requirement to report track and trace information. If you are working for a pharmacy, you are very likely considered a dispenser.
If you are a pharmacist or you are working in a pharmacy and want to better understand your responsibilities under DSCSA, the FDA has a great resource here
.
For everyone else (including physicians and infusion centers), it is important to understand that it is the DSCSA that is driving the adoption of barcodes with serialization information. (More about that later in this article.)
Who is GS1®?
Before we get to barcodes, we need to talk briefly about GS1®. Below is their mission statement along with some “What we do” info:
“Our mission is to increase patient safety, supply chain security and efficiency, traceability and accurate data synchronization in healthcare” (gs1).
GS1® Standards improve the efficiency, safety, and visibility of supply chains across multiple sectors (gs1.org).
In summary, GS1® creates standards, standardizes and hosts data for reporting, and provides education to all users in the supply chain. If you spend a few moments on the GS1® website, you will see that they are involved in a long list of projects that include both healthcare and non-healthcare related industries. For our purposes, we are focused on their participation with the DSCSA and barcoding.
GS1® has an excellent landing page for all their healthcare resources here.
 What is a GTIN?
Before we get into barcodes, we need a little background lesson on GTINs. GTIN stands for Global Trade Item Number and is the globally unique GS1® Identification Number used to identify “trade items” (i.e. products and services that may be priced, ordered, or invoiced at any point in the supply chain).
GTINs are assigned by the brand owner (manufacturer) of the product and are used to identify products as they move through the global supply chain to the end user.
The attributes defined for each GTIN (i.e. size, weight, packaging, etc.) help users to ensure that each GTIN is specific to one very precise trading unit configuration (e.g. a blister of two aspirin tablets; a bottle of 100 aspirin tablets; etc).
In summary, GTINs are unique identifiers printed on medication packaging that help the industry identify and track medications around the world. Products get labeled with GTINs when they are manufactured and should stay with the product through their supply chain journey until they reach the end user.
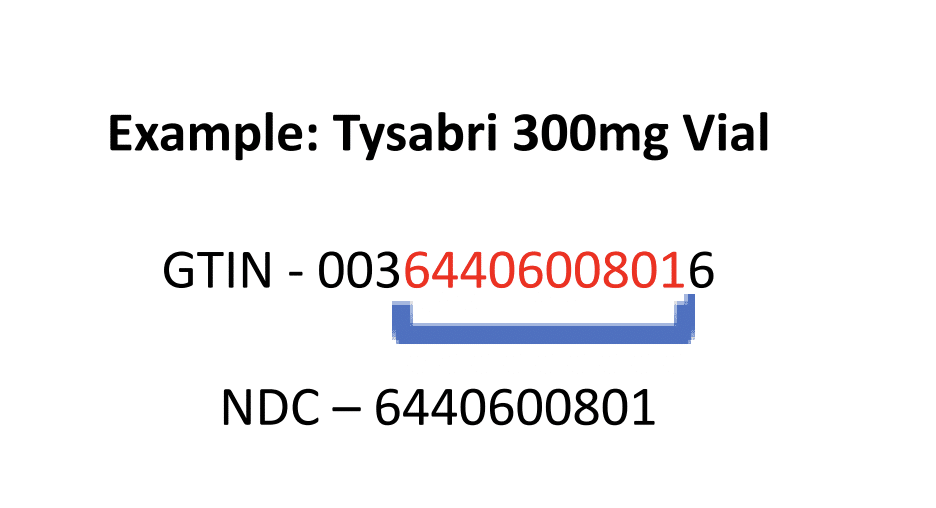
In healthcare, the GTIN number contains the 10-digit product National Drug Code (NDC). If you look at our example below for a single vial of Tysabri 300mg, you will see that the vial packaging is labeled with the GTIN.
What is a GTIN?
Before we get into barcodes, we need a little background lesson on GTINs. GTIN stands for Global Trade Item Number and is the globally unique GS1® Identification Number used to identify “trade items” (i.e. products and services that may be priced, ordered, or invoiced at any point in the supply chain).
GTINs are assigned by the brand owner (manufacturer) of the product and are used to identify products as they move through the global supply chain to the end user.
The attributes defined for each GTIN (i.e. size, weight, packaging, etc.) help users to ensure that each GTIN is specific to one very precise trading unit configuration (e.g. a blister of two aspirin tablets; a bottle of 100 aspirin tablets; etc).
In summary, GTINs are unique identifiers printed on medication packaging that help the industry identify and track medications around the world. Products get labeled with GTINs when they are manufactured and should stay with the product through their supply chain journey until they reach the end user.
In healthcare, the GTIN number contains the 10-digit product National Drug Code (NDC). If you look at our example below for a single vial of Tysabri 300mg, you will see that the vial packaging is labeled with the GTIN.
 DSCSA + GS1 + GTIN
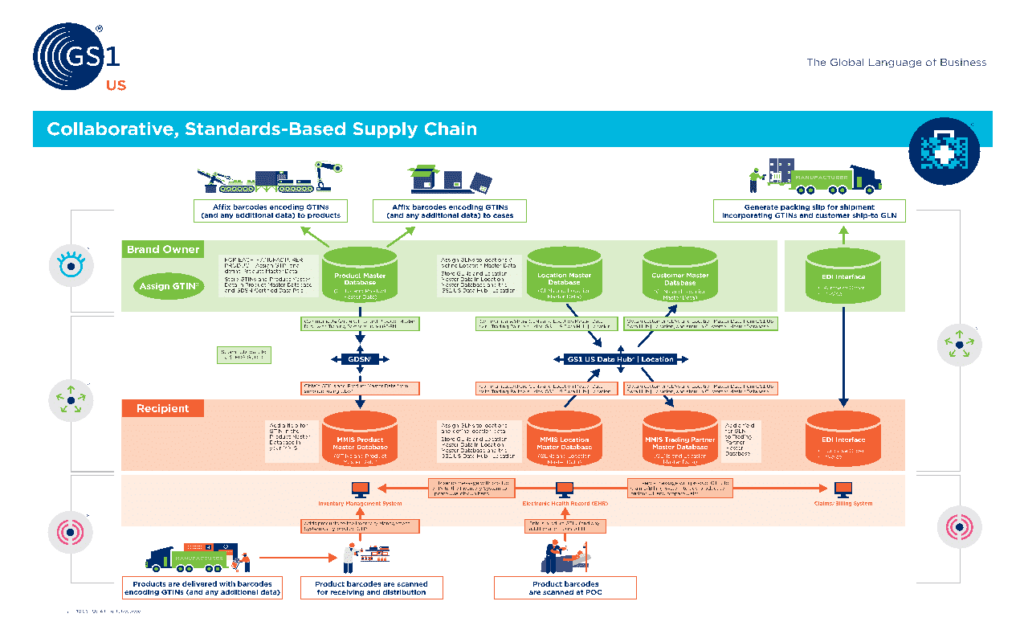
Putting it all together her, these previously covered topics all work together so we have the regulation, standards, and identifiers needed to properly track and identify medications around the globe. If you want to get all the way down into the details, GS1 has a great infographic you see below.
DSCSA + GS1 + GTIN
Putting it all together her, these previously covered topics all work together so we have the regulation, standards, and identifiers needed to properly track and identify medications around the globe. If you want to get all the way down into the details, GS1 has a great infographic you see below.
 If you want to accurately track a medication’s journey all the way from the manufacturer to the end user, everything we covered is absolutely necessary; however, you still need to add in serialization and barcodes to make it all work in a real-world process.
Data Reporting
I am not going to do a deep dive in this article on how all the supply chain data gets reported on all these medications. The important thing to know is that every party under the DSCSA that is required to, or wants to, report must use a standardized format that is managed by GS1 and route that data to a repository known as the Electronic Product Code Information Services (EPCIS). If you imagine EPCIS as a huge database where every step of a medication’s journey is logged, you are thinking in the right direction. Visit EPCIS
to learn more.
Barcode Scanners
Not all barcode scanners are the same and it’s important to understand the differences if you are planning to use barcodes in your operation. There are two main types of scanners.
If you want to accurately track a medication’s journey all the way from the manufacturer to the end user, everything we covered is absolutely necessary; however, you still need to add in serialization and barcodes to make it all work in a real-world process.
Data Reporting
I am not going to do a deep dive in this article on how all the supply chain data gets reported on all these medications. The important thing to know is that every party under the DSCSA that is required to, or wants to, report must use a standardized format that is managed by GS1 and route that data to a repository known as the Electronic Product Code Information Services (EPCIS). If you imagine EPCIS as a huge database where every step of a medication’s journey is logged, you are thinking in the right direction. Visit EPCIS
to learn more.
Barcode Scanners
Not all barcode scanners are the same and it’s important to understand the differences if you are planning to use barcodes in your operation. There are two main types of scanners.
 1D Laser-Based Scanners
These are inexpensive scanners that use a laser to scan 1D linear barcodes
1D Laser-Based Scanners
These are inexpensive scanners that use a laser to scan 1D linear barcodes
 2D Camera-Based Scanners
These are more expensive scanners that use a digital camera to take an image of the barcode. They can be used to scan 1D and 2D barcodes, and can also be a smart device such as a tablet or smartphone app.
Let’s Talk About Barcodes
Most of us already know what a barcode is and how it’s used. Thanks to the invention of self-checkout at grocery stores, most of us now are pretty good at recognizing and locating barcodes on most products.
But not all barcodes are the same, so let’s do a quick barcode overview.
1D Barcode Examples
Below are a few examples of 1D barcodes. 1D barcodes are mostly what you see on retail products. Chances are, there is a product around you right now with one of these printed on it. All of these barcodes below can be read with a laser-based scanner.
2D Camera-Based Scanners
These are more expensive scanners that use a digital camera to take an image of the barcode. They can be used to scan 1D and 2D barcodes, and can also be a smart device such as a tablet or smartphone app.
Let’s Talk About Barcodes
Most of us already know what a barcode is and how it’s used. Thanks to the invention of self-checkout at grocery stores, most of us now are pretty good at recognizing and locating barcodes on most products.
But not all barcodes are the same, so let’s do a quick barcode overview.
1D Barcode Examples
Below are a few examples of 1D barcodes. 1D barcodes are mostly what you see on retail products. Chances are, there is a product around you right now with one of these printed on it. All of these barcodes below can be read with a laser-based scanner.
 UPC-EAN: Used for retail items in mostly POS systems and can include anywhere from 8 to 13 characters
UPC-EAN: Used for retail items in mostly POS systems and can include anywhere from 8 to 13 characters
 ITF-14: Used for higher-level packaging, cases, in distribution
ITF-14: Used for higher-level packaging, cases, in distribution
 GS1 Databar Stacked: More compact code used for small items like loose produce, etc
Below are a few more 1D barcode examples that look the same as the above; however, these examples contain much more information than the previous ones and cannot be read by traditional 1D laser-based scanners.
GS1 Databar Stacked: More compact code used for small items like loose produce, etc
Below are a few more 1D barcode examples that look the same as the above; however, these examples contain much more information than the previous ones and cannot be read by traditional 1D laser-based scanners.
 GS1 DataBar Truncated: Used for very small item identification. Typically, unit-dose pharmaceuticals.
GS1 DataBar Truncated: Used for very small item identification. Typically, unit-dose pharmaceuticals.
 GS1 128: Used for higher-level packaging, cases, pallets, in distribution
GS1 128: Used for higher-level packaging, cases, pallets, in distribution
 GS1 DataBar Expanded Stacked: Contains additional information such as expiration, lot, etc.
2D Barcodes
Most of the barcodes we are concerned with for this article will be 2D barcodes. 2D barcodes are different as they can contain much more information than 1D barcodes and they cannot be read with traditional laser-based scanners.
2D Barcode Examples
GS1 DataBar Expanded Stacked: Contains additional information such as expiration, lot, etc.
2D Barcodes
Most of the barcodes we are concerned with for this article will be 2D barcodes. 2D barcodes are different as they can contain much more information than 1D barcodes and they cannot be read with traditional laser-based scanners.
2D Barcode Examples
 QR Codes: Used mostly by consumers using a mobile device to link a product to information found on the internet
QR Codes: Used mostly by consumers using a mobile device to link a product to information found on the internet
 GS1 2D Datamatrix: The official barcode for our discussion
GS1 2D DataMatrix
Our hero of the discussion today is the GS1 2D DataMatrix code. This is the primary workhorse of the DSCSA.
GS1 2D Datamatrix: The official barcode for our discussion
GS1 2D DataMatrix
Our hero of the discussion today is the GS1 2D DataMatrix code. This is the primary workhorse of the DSCSA.
 GS1 2D DataMatrix – Advantages
GS1 2D DataMatrix – Advantages

 Other Barcodes Used in DSCSA
There are many other types of barcodes used in the DSCSA workflow as shown below; however for our discussion, we will focus on the GS1 2D DataMatrix. If you want to nerd out on all the other barcodes, GS1 has a great graphic (shown below) that you can review.
Other Barcodes Used in DSCSA
There are many other types of barcodes used in the DSCSA workflow as shown below; however for our discussion, we will focus on the GS1 2D DataMatrix. If you want to nerd out on all the other barcodes, GS1 has a great graphic (shown below) that you can review.
 GS1 2D Datamatrix Codes Continued…
The GS1 Datamatrix codes used on medications will contain some very specific information about the label medication. It is important to understand both the format and content of this information if you are going to try and leverage these in your operation.
GS1 2D Datamatrix Codes Continued…
The GS1 Datamatrix codes used on medications will contain some very specific information about the label medication. It is important to understand both the format and content of this information if you are going to try and leverage these in your operation.

 Most printed datamatrix codes will have human-readable text below that shows the information contained in the code. When scanned, the output of the code will be a single concatenated string of characters with prefixes (separators) that identify each string. For pharmaceuticals, the prefix/format above of 01 (GTIN), 21 (Serial), 10 (Lot), and 17 (Expiration date) is the standard.
If you were to scan the above code, the output to your device, would look like this below:
010950400005910121190678118111053GS117200331
Most printed datamatrix codes will have human-readable text below that shows the information contained in the code. When scanned, the output of the code will be a single concatenated string of characters with prefixes (separators) that identify each string. For pharmaceuticals, the prefix/format above of 01 (GTIN), 21 (Serial), 10 (Lot), and 17 (Expiration date) is the standard.
If you were to scan the above code, the output to your device, would look like this below:
010950400005910121190678118111053GS117200331

 It is very important to note that today, the code is printed on the package in most cases, not the actual container/vial/syringe/bag. This is something that can have an operational impact on your business if at some point in the workflow there are any steps in which the container is separated from its packaging. For my infusion pharmacy audience, this is very common when moving medications into sterile clean rooms. If you are going to capture the serialized barcode information from a medication, you must do so before that medication container is separated from its packaging.
For others, it might be common practice for inventory managers/nurses, etc. to remove vials from their packaging prior to storing them in bins on the shelf or in a refrigerator. You might want to look at this before putting any process into place that involves capturing barcode information.
At this point, you might be thinking that all this information is fascinating, but why should you care?
A few reasons below…
It is very important to note that today, the code is printed on the package in most cases, not the actual container/vial/syringe/bag. This is something that can have an operational impact on your business if at some point in the workflow there are any steps in which the container is separated from its packaging. For my infusion pharmacy audience, this is very common when moving medications into sterile clean rooms. If you are going to capture the serialized barcode information from a medication, you must do so before that medication container is separated from its packaging.
For others, it might be common practice for inventory managers/nurses, etc. to remove vials from their packaging prior to storing them in bins on the shelf or in a refrigerator. You might want to look at this before putting any process into place that involves capturing barcode information.
At this point, you might be thinking that all this information is fascinating, but why should you care?
A few reasons below…
This can and does improve patient safety
You can leverage this system to improve and modernize your inventory management and accounting processes
Barcodes Improve Patient Safety
There is real application of this technology today that you can use to improve patient safety. The most obvious way is by preventing medication errors. Now that each medication has a unique, identifiable barcode, many technology applications can and should give clinical users the ability to confirm medications prior to and during their administration to a patient.
If you are a pharmacist/pharmacy, the FDA put out an article discussing this here.
In addition, every year there are thousands of medication recalls. Many of these go unnoticed by healthcare providers due to lapses in communication by the recalling party. The advent of these barcodes across all medications means that everyone can participate in lot level, serialized recalls so that no recalled medication should ever be administered to a patient.
Barcodes Improve Inventory Management
Prior to having serialized GS1 Datamatrix codes on medications, anyone handling the supply chain and inventory of medications had to rely on batch identification for their inventory counts. This means the lowest level of identity for a medication container came down to NDC, lot, and expiration. Since those three values can be the same for many of the same containers, it was difficult to track the containers individually in any system.
By having a unique identifier on every individual container, systems can now track and keep up with every container’s location and movement through the system. In any inventory system, this is a capability that is necessary to keep a complete record of items.
Anytime I speak on this topic, I poll the audience to ask how much money they believe they have sitting on their shelf and in their refrigerator back at their office. The answers typically come back in the hundreds of thousands up to the tens of millions.
If you are the person who is responsible for these medications (especially if your name is on the credit app!), you should always be absolutely certain about how many vials you purchased, received, documented on a treatment, and were billed on a claim and will be invoiced and paid for.
Reconciliation of inventory is critical for any operation. I call it balancing the big 6 in the order below.
PO → Packing Slip→ Treatment Documentation → Claim → Payment → Invoice
If you are one of our infusion center friends, these unique barcodes are very useful for eliminating the chance that a Buy & Bill and specialty pharmacy medication get inadvertently swapped on the wrong patient. I am sure that never happens to anyone reading this, but just in case, I wanted to mention it here. There are technology solutions available to you today that utilize these barcodes.
I am certainly not the first person to have these ideas. It is common, especially in larger hospitals and medical institutions, to take advantage of applications that utilize these barcodes in their inventory and clinical workflows.
The best applications on the market today offer your team the ability to incorporate these barcodes into your workflow. I might know of one great software in particular that is really good for this if you ask (hint: it’s WeInfuse).
DIY Works Too
Although I am biased, the truth is that you don’t actually have to have a specialized application solution to take advantage of this technology today. There are ways that your team can incorporate these barcodes into your current workflows if you have a bit of technical know-how and are willing to do a little extra manual work.
As mentioned earlier, any 2D barcode can be read with a camera-based 2D scanner, a smartphone, or a camera-equipped tablet device. The trick is to build in some level of programming or formular to help separate the barcode’s various parts for your users.
Most barcode scanners interface with computers by mimicking the keyboard via USB or bluetooth. Basically, when the barcode is scanned, the scanner sends in messages to the computer the same as if you typed the numbers in yourself. Most will also allow some level of programming that a user can access to do this automatically. The best quality scanners come from companies like Zebra and do this very well. In the below example, I did some light programming on a Zebra scanner to add in some separators on the various parts of a GS1 Datamatrix code so that some spreadsheet formulas could be used to break out the GTIN, NDC, lot, expiration, and serial number for any scanned code.
 Any EHR/EMR, etc. text field can have this information put in by using a scanner, or your team can use a spreadsheet like the one above to track inventory in and out the door and/or document the inventories use/administration to an individual patient.
What Does the Future Hold?
At the time of this writing, most of the DSCSA is being completed and ends with its provisions enforced from the manufacturer down to the dispenser. Physician providers are currently exempt from most of the DSCSA data tracking and reporting provisions currently. This means that (at least for now) there is no requirement for physicians to track a medication’s usage down to the actual patient that received the medication.
When will this be required? I have written the FDA on the topic a few times, and their response each time is that it’s not required today, and they continue to discuss that moving forward. My instinct tells me that it will take another event in the space to trigger this requirement. If anyone recalls the sterile compounding tragedy in New England a few years back, this event created a renewed focus for more regulation. I always say that “political chills cause Congressional bills.”
Another prediction I will make is very unpopular but meaningful. Now that all of the medications are uniquely identifiable and reported back to a master dataset, it makes total sense to me that both government and private health insurance payers would move to utilize this data in order to prevent insurance fraud. There is plenty of room on an electronic claim to include serial numbers of administered medications. With this information, it would be fairly easy for any payer to confirm if the medications being billed for their members were authentic and prevent any duplication of that billing from any/multiple providers (something to think about.)
It’s Not If but When
It never pays to be at the back of the pack and do the last-minute catch-up needed for regulatory deadlines. The DSCSA is here to stay and will certainly continue to expand as more of the industry adopts this level of tracking.
Even if the current and future regulations do not concern you, there are many compelling reasons to understand and utilize serialized barcode information in your workflows. I hope this article has helped you and your team better understand how and why these regulations and technologies exist today. At the end of the day, most of us want to do meaningful work to improve our processes so that our team members can be more efficient and satisfied, and spend more time focused on our customers and patients.
If this article was helpful, or you have used barcodes in your operations in an innovative way, I would love to hear your stories. You can write to info@weinfuse.com or use the contact form on our website at weinfuse.com
.
Any EHR/EMR, etc. text field can have this information put in by using a scanner, or your team can use a spreadsheet like the one above to track inventory in and out the door and/or document the inventories use/administration to an individual patient.
What Does the Future Hold?
At the time of this writing, most of the DSCSA is being completed and ends with its provisions enforced from the manufacturer down to the dispenser. Physician providers are currently exempt from most of the DSCSA data tracking and reporting provisions currently. This means that (at least for now) there is no requirement for physicians to track a medication’s usage down to the actual patient that received the medication.
When will this be required? I have written the FDA on the topic a few times, and their response each time is that it’s not required today, and they continue to discuss that moving forward. My instinct tells me that it will take another event in the space to trigger this requirement. If anyone recalls the sterile compounding tragedy in New England a few years back, this event created a renewed focus for more regulation. I always say that “political chills cause Congressional bills.”
Another prediction I will make is very unpopular but meaningful. Now that all of the medications are uniquely identifiable and reported back to a master dataset, it makes total sense to me that both government and private health insurance payers would move to utilize this data in order to prevent insurance fraud. There is plenty of room on an electronic claim to include serial numbers of administered medications. With this information, it would be fairly easy for any payer to confirm if the medications being billed for their members were authentic and prevent any duplication of that billing from any/multiple providers (something to think about.)
It’s Not If but When
It never pays to be at the back of the pack and do the last-minute catch-up needed for regulatory deadlines. The DSCSA is here to stay and will certainly continue to expand as more of the industry adopts this level of tracking.
Even if the current and future regulations do not concern you, there are many compelling reasons to understand and utilize serialized barcode information in your workflows. I hope this article has helped you and your team better understand how and why these regulations and technologies exist today. At the end of the day, most of us want to do meaningful work to improve our processes so that our team members can be more efficient and satisfied, and spend more time focused on our customers and patients.
If this article was helpful, or you have used barcodes in your operations in an innovative way, I would love to hear your stories. You can write to info@weinfuse.com or use the contact form on our website at weinfuse.com
.
 Why Do We Need the DSCSA?
According to the FDA, the DSCSA was created to…
Why Do We Need the DSCSA?
According to the FDA, the DSCSA was created to…
- Help protect consumers from exposure to drugs that may be counterfeit, stolen, contaminated, or otherwise harmful.
- GS1® is a neutral, not-for-profit, global organization that develops and maintains the most widely-used supply chain standards system in the world (gs1.org)
 What is a GTIN?
Before we get into barcodes, we need a little background lesson on GTINs. GTIN stands for Global Trade Item Number and is the globally unique GS1® Identification Number used to identify “trade items” (i.e. products and services that may be priced, ordered, or invoiced at any point in the supply chain).
GTINs are assigned by the brand owner (manufacturer) of the product and are used to identify products as they move through the global supply chain to the end user.
The attributes defined for each GTIN (i.e. size, weight, packaging, etc.) help users to ensure that each GTIN is specific to one very precise trading unit configuration (e.g. a blister of two aspirin tablets; a bottle of 100 aspirin tablets; etc).
In summary, GTINs are unique identifiers printed on medication packaging that help the industry identify and track medications around the world. Products get labeled with GTINs when they are manufactured and should stay with the product through their supply chain journey until they reach the end user.
In healthcare, the GTIN number contains the 10-digit product National Drug Code (NDC). If you look at our example below for a single vial of Tysabri 300mg, you will see that the vial packaging is labeled with the GTIN.
What is a GTIN?
Before we get into barcodes, we need a little background lesson on GTINs. GTIN stands for Global Trade Item Number and is the globally unique GS1® Identification Number used to identify “trade items” (i.e. products and services that may be priced, ordered, or invoiced at any point in the supply chain).
GTINs are assigned by the brand owner (manufacturer) of the product and are used to identify products as they move through the global supply chain to the end user.
The attributes defined for each GTIN (i.e. size, weight, packaging, etc.) help users to ensure that each GTIN is specific to one very precise trading unit configuration (e.g. a blister of two aspirin tablets; a bottle of 100 aspirin tablets; etc).
In summary, GTINs are unique identifiers printed on medication packaging that help the industry identify and track medications around the world. Products get labeled with GTINs when they are manufactured and should stay with the product through their supply chain journey until they reach the end user.
In healthcare, the GTIN number contains the 10-digit product National Drug Code (NDC). If you look at our example below for a single vial of Tysabri 300mg, you will see that the vial packaging is labeled with the GTIN.
 DSCSA + GS1 + GTIN
Putting it all together her, these previously covered topics all work together so we have the regulation, standards, and identifiers needed to properly track and identify medications around the globe. If you want to get all the way down into the details, GS1 has a great infographic you see below.
DSCSA + GS1 + GTIN
Putting it all together her, these previously covered topics all work together so we have the regulation, standards, and identifiers needed to properly track and identify medications around the globe. If you want to get all the way down into the details, GS1 has a great infographic you see below.
 If you want to accurately track a medication’s journey all the way from the manufacturer to the end user, everything we covered is absolutely necessary; however, you still need to add in serialization and barcodes to make it all work in a real-world process.
Data Reporting
I am not going to do a deep dive in this article on how all the supply chain data gets reported on all these medications. The important thing to know is that every party under the DSCSA that is required to, or wants to, report must use a standardized format that is managed by GS1 and route that data to a repository known as the Electronic Product Code Information Services (EPCIS). If you imagine EPCIS as a huge database where every step of a medication’s journey is logged, you are thinking in the right direction. Visit EPCIS
to learn more.
Barcode Scanners
Not all barcode scanners are the same and it’s important to understand the differences if you are planning to use barcodes in your operation. There are two main types of scanners.
If you want to accurately track a medication’s journey all the way from the manufacturer to the end user, everything we covered is absolutely necessary; however, you still need to add in serialization and barcodes to make it all work in a real-world process.
Data Reporting
I am not going to do a deep dive in this article on how all the supply chain data gets reported on all these medications. The important thing to know is that every party under the DSCSA that is required to, or wants to, report must use a standardized format that is managed by GS1 and route that data to a repository known as the Electronic Product Code Information Services (EPCIS). If you imagine EPCIS as a huge database where every step of a medication’s journey is logged, you are thinking in the right direction. Visit EPCIS
to learn more.
Barcode Scanners
Not all barcode scanners are the same and it’s important to understand the differences if you are planning to use barcodes in your operation. There are two main types of scanners.
 1D Laser-Based Scanners
These are inexpensive scanners that use a laser to scan 1D linear barcodes
1D Laser-Based Scanners
These are inexpensive scanners that use a laser to scan 1D linear barcodes
 2D Camera-Based Scanners
These are more expensive scanners that use a digital camera to take an image of the barcode. They can be used to scan 1D and 2D barcodes, and can also be a smart device such as a tablet or smartphone app.
Let’s Talk About Barcodes
Most of us already know what a barcode is and how it’s used. Thanks to the invention of self-checkout at grocery stores, most of us now are pretty good at recognizing and locating barcodes on most products.
But not all barcodes are the same, so let’s do a quick barcode overview.
1D Barcode Examples
Below are a few examples of 1D barcodes. 1D barcodes are mostly what you see on retail products. Chances are, there is a product around you right now with one of these printed on it. All of these barcodes below can be read with a laser-based scanner.
2D Camera-Based Scanners
These are more expensive scanners that use a digital camera to take an image of the barcode. They can be used to scan 1D and 2D barcodes, and can also be a smart device such as a tablet or smartphone app.
Let’s Talk About Barcodes
Most of us already know what a barcode is and how it’s used. Thanks to the invention of self-checkout at grocery stores, most of us now are pretty good at recognizing and locating barcodes on most products.
But not all barcodes are the same, so let’s do a quick barcode overview.
1D Barcode Examples
Below are a few examples of 1D barcodes. 1D barcodes are mostly what you see on retail products. Chances are, there is a product around you right now with one of these printed on it. All of these barcodes below can be read with a laser-based scanner.
 UPC-EAN: Used for retail items in mostly POS systems and can include anywhere from 8 to 13 characters
UPC-EAN: Used for retail items in mostly POS systems and can include anywhere from 8 to 13 characters
 ITF-14: Used for higher-level packaging, cases, in distribution
ITF-14: Used for higher-level packaging, cases, in distribution
 GS1 Databar Stacked: More compact code used for small items like loose produce, etc
Below are a few more 1D barcode examples that look the same as the above; however, these examples contain much more information than the previous ones and cannot be read by traditional 1D laser-based scanners.
GS1 Databar Stacked: More compact code used for small items like loose produce, etc
Below are a few more 1D barcode examples that look the same as the above; however, these examples contain much more information than the previous ones and cannot be read by traditional 1D laser-based scanners.
 GS1 128: Used for higher-level packaging, cases, pallets, in distribution
GS1 128: Used for higher-level packaging, cases, pallets, in distribution
 GS1 DataBar Expanded Stacked: Contains additional information such as expiration, lot, etc.
2D Barcodes
Most of the barcodes we are concerned with for this article will be 2D barcodes. 2D barcodes are different as they can contain much more information than 1D barcodes and they cannot be read with traditional laser-based scanners.
2D Barcode Examples
GS1 DataBar Expanded Stacked: Contains additional information such as expiration, lot, etc.
2D Barcodes
Most of the barcodes we are concerned with for this article will be 2D barcodes. 2D barcodes are different as they can contain much more information than 1D barcodes and they cannot be read with traditional laser-based scanners.
2D Barcode Examples
 QR Codes: Used mostly by consumers using a mobile device to link a product to information found on the internet
QR Codes: Used mostly by consumers using a mobile device to link a product to information found on the internet
 GS1 2D Datamatrix: The official barcode for our discussion
GS1 2D DataMatrix
Our hero of the discussion today is the GS1 2D DataMatrix code. This is the primary workhorse of the DSCSA.
GS1 2D Datamatrix: The official barcode for our discussion
GS1 2D DataMatrix
Our hero of the discussion today is the GS1 2D DataMatrix code. This is the primary workhorse of the DSCSA.
 GS1 2D DataMatrix – Advantages
GS1 2D DataMatrix – Advantages
- Allows encoding of much more data in smaller space
- More flexible to print or engrave on a variety of products
- More robust for readability, error detection, etc

 Other Barcodes Used in DSCSA
There are many other types of barcodes used in the DSCSA workflow as shown below; however for our discussion, we will focus on the GS1 2D DataMatrix. If you want to nerd out on all the other barcodes, GS1 has a great graphic (shown below) that you can review.
Other Barcodes Used in DSCSA
There are many other types of barcodes used in the DSCSA workflow as shown below; however for our discussion, we will focus on the GS1 2D DataMatrix. If you want to nerd out on all the other barcodes, GS1 has a great graphic (shown below) that you can review.
 GS1 2D Datamatrix Codes Continued…
The GS1 Datamatrix codes used on medications will contain some very specific information about the label medication. It is important to understand both the format and content of this information if you are going to try and leverage these in your operation.
GS1 2D Datamatrix Codes Continued…
The GS1 Datamatrix codes used on medications will contain some very specific information about the label medication. It is important to understand both the format and content of this information if you are going to try and leverage these in your operation.

 Most printed datamatrix codes will have human-readable text below that shows the information contained in the code. When scanned, the output of the code will be a single concatenated string of characters with prefixes (separators) that identify each string. For pharmaceuticals, the prefix/format above of 01 (GTIN), 21 (Serial), 10 (Lot), and 17 (Expiration date) is the standard.
If you were to scan the above code, the output to your device, would look like this below:
010950400005910121190678118111053GS117200331
Most printed datamatrix codes will have human-readable text below that shows the information contained in the code. When scanned, the output of the code will be a single concatenated string of characters with prefixes (separators) that identify each string. For pharmaceuticals, the prefix/format above of 01 (GTIN), 21 (Serial), 10 (Lot), and 17 (Expiration date) is the standard.
If you were to scan the above code, the output to your device, would look like this below:
010950400005910121190678118111053GS117200331
Where Are These Barcodes?
Now that we are all up to date on the GS1 Datamatrix barcode, we need to know where to find them. Per DSCSA, all the following must have a 2D GS1 Datamatrix code printed on them from the manufacturer. “Therefore, manufacturers and repackagers must determine the smallest individual saleable unit product configuration that they intend to be sold to the dispenser, and affix or imprint a product identifier (included in a 2-dimensional data matrix barcode) to that package” (fda.gov). Basically, whatever you get delivered today by your wholesaler or distributor should have a 2D GS1 Datamatrix code printed on the package it came in. I have included a few photos below of actual codes printed on their packages/boxes.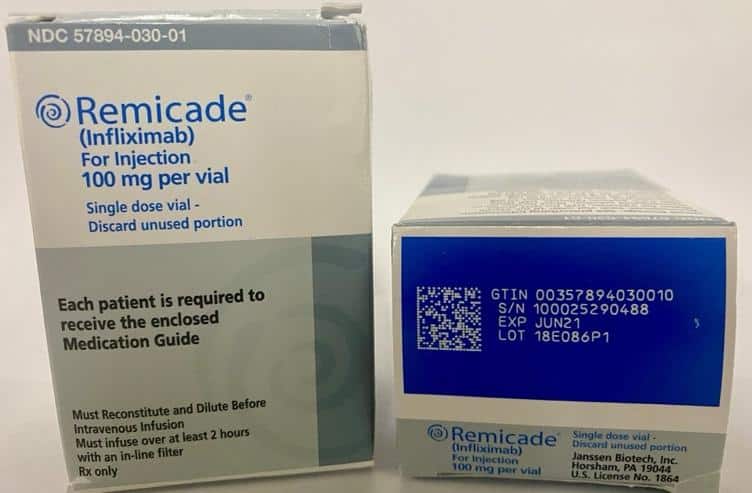
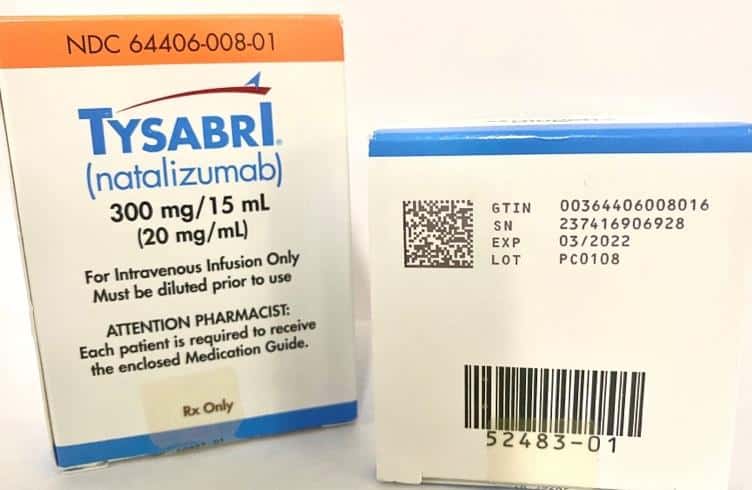 It is very important to note that today, the code is printed on the package in most cases, not the actual container/vial/syringe/bag. This is something that can have an operational impact on your business if at some point in the workflow there are any steps in which the container is separated from its packaging. For my infusion pharmacy audience, this is very common when moving medications into sterile clean rooms. If you are going to capture the serialized barcode information from a medication, you must do so before that medication container is separated from its packaging.
For others, it might be common practice for inventory managers/nurses, etc. to remove vials from their packaging prior to storing them in bins on the shelf or in a refrigerator. You might want to look at this before putting any process into place that involves capturing barcode information.
At this point, you might be thinking that all this information is fascinating, but why should you care?
A few reasons below…
It is very important to note that today, the code is printed on the package in most cases, not the actual container/vial/syringe/bag. This is something that can have an operational impact on your business if at some point in the workflow there are any steps in which the container is separated from its packaging. For my infusion pharmacy audience, this is very common when moving medications into sterile clean rooms. If you are going to capture the serialized barcode information from a medication, you must do so before that medication container is separated from its packaging.
For others, it might be common practice for inventory managers/nurses, etc. to remove vials from their packaging prior to storing them in bins on the shelf or in a refrigerator. You might want to look at this before putting any process into place that involves capturing barcode information.
At this point, you might be thinking that all this information is fascinating, but why should you care?
A few reasons below…
- You might want to know in order to comply with DSCSA
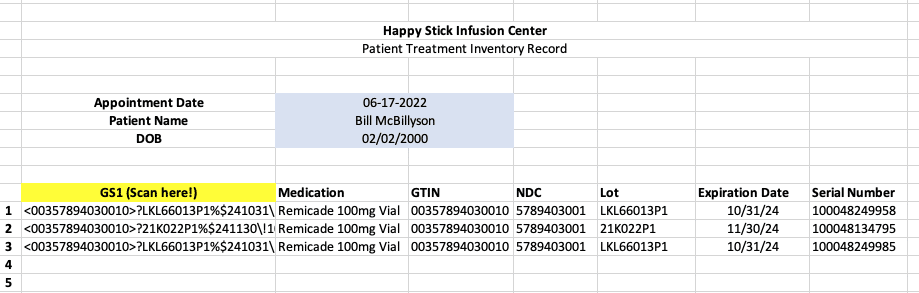 Any EHR/EMR, etc. text field can have this information put in by using a scanner, or your team can use a spreadsheet like the one above to track inventory in and out the door and/or document the inventories use/administration to an individual patient.
What Does the Future Hold?
At the time of this writing, most of the DSCSA is being completed and ends with its provisions enforced from the manufacturer down to the dispenser. Physician providers are currently exempt from most of the DSCSA data tracking and reporting provisions currently. This means that (at least for now) there is no requirement for physicians to track a medication’s usage down to the actual patient that received the medication.
When will this be required? I have written the FDA on the topic a few times, and their response each time is that it’s not required today, and they continue to discuss that moving forward. My instinct tells me that it will take another event in the space to trigger this requirement. If anyone recalls the sterile compounding tragedy in New England a few years back, this event created a renewed focus for more regulation. I always say that “political chills cause Congressional bills.”
Another prediction I will make is very unpopular but meaningful. Now that all of the medications are uniquely identifiable and reported back to a master dataset, it makes total sense to me that both government and private health insurance payers would move to utilize this data in order to prevent insurance fraud. There is plenty of room on an electronic claim to include serial numbers of administered medications. With this information, it would be fairly easy for any payer to confirm if the medications being billed for their members were authentic and prevent any duplication of that billing from any/multiple providers (something to think about.)
It’s Not If but When
It never pays to be at the back of the pack and do the last-minute catch-up needed for regulatory deadlines. The DSCSA is here to stay and will certainly continue to expand as more of the industry adopts this level of tracking.
Even if the current and future regulations do not concern you, there are many compelling reasons to understand and utilize serialized barcode information in your workflows. I hope this article has helped you and your team better understand how and why these regulations and technologies exist today. At the end of the day, most of us want to do meaningful work to improve our processes so that our team members can be more efficient and satisfied, and spend more time focused on our customers and patients.
If this article was helpful, or you have used barcodes in your operations in an innovative way, I would love to hear your stories. You can write to info@weinfuse.com or use the contact form on our website at weinfuse.com
.
Any EHR/EMR, etc. text field can have this information put in by using a scanner, or your team can use a spreadsheet like the one above to track inventory in and out the door and/or document the inventories use/administration to an individual patient.
What Does the Future Hold?
At the time of this writing, most of the DSCSA is being completed and ends with its provisions enforced from the manufacturer down to the dispenser. Physician providers are currently exempt from most of the DSCSA data tracking and reporting provisions currently. This means that (at least for now) there is no requirement for physicians to track a medication’s usage down to the actual patient that received the medication.
When will this be required? I have written the FDA on the topic a few times, and their response each time is that it’s not required today, and they continue to discuss that moving forward. My instinct tells me that it will take another event in the space to trigger this requirement. If anyone recalls the sterile compounding tragedy in New England a few years back, this event created a renewed focus for more regulation. I always say that “political chills cause Congressional bills.”
Another prediction I will make is very unpopular but meaningful. Now that all of the medications are uniquely identifiable and reported back to a master dataset, it makes total sense to me that both government and private health insurance payers would move to utilize this data in order to prevent insurance fraud. There is plenty of room on an electronic claim to include serial numbers of administered medications. With this information, it would be fairly easy for any payer to confirm if the medications being billed for their members were authentic and prevent any duplication of that billing from any/multiple providers (something to think about.)
It’s Not If but When
It never pays to be at the back of the pack and do the last-minute catch-up needed for regulatory deadlines. The DSCSA is here to stay and will certainly continue to expand as more of the industry adopts this level of tracking.
Even if the current and future regulations do not concern you, there are many compelling reasons to understand and utilize serialized barcode information in your workflows. I hope this article has helped you and your team better understand how and why these regulations and technologies exist today. At the end of the day, most of us want to do meaningful work to improve our processes so that our team members can be more efficient and satisfied, and spend more time focused on our customers and patients.
If this article was helpful, or you have used barcodes in your operations in an innovative way, I would love to hear your stories. You can write to info@weinfuse.com or use the contact form on our website at weinfuse.com
. 



